Medical Megamania
Wednesday, March 6, 2013
We have moved to medimegamania.com
Saturday, January 5, 2013
India’s first intestine transplantation by Dr.Arvinder Singh Soin
Transplantation medicine is one of the fast growing disciplines of medical science in India.On 24th Nov 2012 first intestine transplantation was conducted on Himanshu Singh, a techie at Medanta-the Medicity, Gurgaon by a team of 30 doctors lead by Dr.Arvider Singh Soin ,pioneer in the field of hepatobiliary transplantation.
3 years ago Himanshu Singh had suffered gangrene of small intestine which led to resection of whole of small intestine except for 28cm length. He was kept on Total Parenteral Nutrition (TPN) for a long period and he suffered the complications of TPN mainly, infections.Then on 24th Nov 2012 a cadaver donor small intestine transplantation was conducted successfully and Himanshu singh is recovering gradually now.
Dr.Arvinder Singh Soin , a hepatobiliary surgeon and an eminent liver transplantation surgeon, lead the team of doctors who conducted this transplantation. Dr.Soin has conducted more than 1000 liver transplantations in India which is highest in India and he has achieved so many firsts in the field of transplantation in India. He was awarded the ‘Padma Shri’ in 2010 for his contribution to the field of medical science.
This feat achieved at the Medanta the Medicity gives new hope to so many people with malabsorption syndrome ,inflammatory syndromes etc.
For details visit:
Monday, December 17, 2012
Raxibacumab- Approved for the treatment of inhalational Anthrax

The U.S FDA today approved raxibacumab a monoclonal antibody in the prophylaxis and treatment of inhalational anthrax. Anthrax is a disease caused by Bacillus anthracis bacteria.Inhalational form is also known as wool sorter’s disease as workers in wool industry inhale the spores of bacillus anthracis while sorting out the wool.Anthrax has also been tried to use as a weapon of bioterrorism.
Raxibacumab is a chimeric monoclonal antibody targeting the protective antigen (PA) component of the lethal toxin of Bacillus anthracis. In case of bioterrorism by the use of anthrax spores, raxibacumab is available along with the antibiotics.
More animals treated with raxibacumab lived compared to animals treated with placebo. Sixty-four percent of animals in the monkey study and 44 percent of animals in one rabbit study receiving the 40 milligrams per kilogram dose of raxibacumab survived exposure to anthrax, compared with none in the placebo groups. All surviving animals developed toxin-neutralizing antibodies. Another study in rabbits showed that 82 percent of animals treated with antibiotics and raxibacumab survived exposure to anthrax compared with 65 percent of animals receiving antibiotic treatment alone.
The safety of raxibacumab was evaluated in 326 healthy human volunteers. Common side effects included rash, extremity pain, itching and drowsiness.
Raxibacumab was developed by Rockville, Md.-based Human Genome Sciences, in conjunction with the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority. Human Genome Sciences has since been acquired by GlaxoSmithKline.
Source:http://http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332341.htm
FDA approves Signifor® for the treatment of Cushing’s Disease

Cushing’s disease is a condition caused by pituitary adenoma ,which leads to overstimulation of adrenal glands and overproduction of cortisol. Leading to spectrum of signs symptoms including central obesity, buffalo hump,purple striae, altered diurnal variation, hyperglycemia,hypertension hirsutism and many more. There is one more entity called Cushing’s syndrome which has same range of signs and symptoms but caused due to other peripheral causes most commonly being iatrogenic i.e steroid administration.
Usually Cushing's disease is due to pituitary adenoma or a small tumour which is removed surgically. In patients in whom it cannot removed surgically, Signifor can be given. It is a somatostatin analogue which acts mainly on somatostatin receptor 5.
FDA approved Signifor based on the results of PASPORT-CUSHINGS (PASireotide clinical trial PORTfolio - CUSHING'S disease).Results from the PASPORT-CUSHINGS study found that a decrease in mean urinary-free cortisol (UFC), the key measure of biochemical control of the disease, was sustained during the treatment period in most patients with a subset of patients reaching normal levels. The study also showed that certain clinical manifestations of Cushing's disease tended to improve.
Signifor is given subcutaneously. The important side effects being hyperglycemia, diarrhea, nausea, abdominal pain, and gallstones.
Source:http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332351.htm
Friday, November 30, 2012
Cometriq - Approved for Medullary thyroid cancer
The U.S FDA on 29th nov 2012 approved Cometriq (cabozantinib) , inhibitor of the tyrosine kinases c-Met and VEGFR2 effective in the treatment of progressive, metastatic Medullary Thyroid Carcinoma (MTC).
It is the second drug approved by the US FDA for the treatment of MTC , fist being Caprelsa (vandetanib). MTC is a cancer arising from parafollicular C cells in the thyroid gland ,which normally secrete calcitonin - calcium lowering hormone. MTC is a cancer difficult to treat especially if it has metastasized. MTC diagnosed early are treated with total thyroidectomy.MTC is associated with familial cancer syndromes MEN(Multiple endocrine neoplasia) 2A and 2B. It is a rare type of thyroid cance with amounting to only 4% of all thyroid cancers.
With the approval of Cometriq patients with advanced MTC will have a new ray of hope.
Source : http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm330143.htm
Friday, July 13, 2012
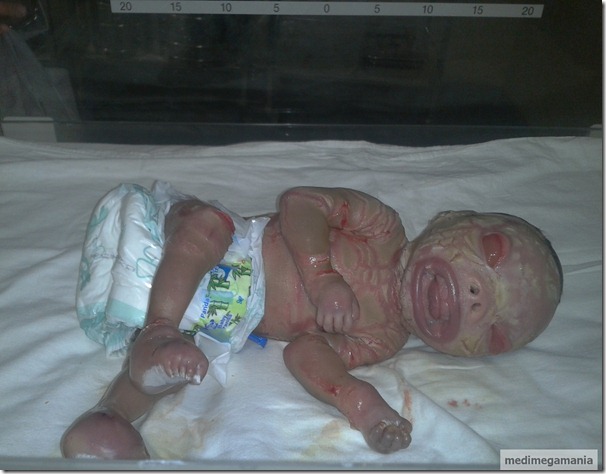
Collodion Baby
Lamellar ichthyosis (LI) is an autosomal recessive disorder that is apparent at birth and is present throughout life. The newborn is born encased in a collodion membrane that sheds within 10-14 days. The shedding of the membrane reveals generalized scaling with variable redness of the skin. The scaling may be fine or platelike, resembling fish skin. Although the disorder is not life threatening, it is quite disfiguring and causes considerable psychological stress to affected patients. Ectropion will be present. There will be macroglossia.
Patients with lamellar ichthyosis have accelerated epidermal turnover with proliferative hyperkeratosis, in contrast to retention hyperkeratosis. This involves a mutation in the gene for transglutaminase 1 (TGM1). The transglutaminase 1 enzyme is involved in the formation of the cornified cell envelope. The formation of the cornified cell envelope is an essential scaffold upon which normal intercellular lipid layer formation in the stratum corneum occurs. Thus, mutations in the TGM1 secondarily cause defects in the intercellular lipid layers in the stratum corneum, leading to defective barrier function of the stratum corneum and to the ichthyotic phenotype seen in lamellar ichthyosis patients and in transglutaminase 1 knockout mice. How much a defective cornified cell envelope alone contributes to the barrier abnormality in ichthyoses remains unclear.[1]
To date, 6 genes for lamellar ichthyosis have been localized and 5 of them identified, as follows[2] :
-
TGM1 (14q11)
-
ABCA12 (2q34)
-
19p12-q12
-
19p13
-
ALOXE3-ALOX12B (17p13)
-
ichthyin (5q33)
In the neonatal period, following the shedding of the collodion membrane, the newborn is at risk for secondary sepsis and hypernatremic dehydration.
As the child ages, the hyperkeratosis can interfere with normal sweat gland function, which can predispose to heat intolerance and possible heat shock. Ectropion may result in the inability to fully close the eyelids and can cause exposure keratitis.
Recently , a case was reported in ESI Post Graduate Institute of Medical Sciences and Research, Rajajinagar,Bangalore.
Picture Courtesy : Dr.Tejaswini Hiremath MS OBG(std) ESIPGIMSR,Bangalore, India
Ref: 1. http://emedicine.medscape.com/article/1111300-overview
Tuesday, July 10, 2012
The Amazing Spider Surgery!
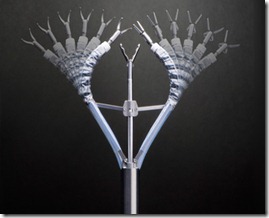
In the conventional laparoscopic surgery there are at least 4 to 5 ports/small incisions around the umbilicus for the insertion of various instruments including a camera.
But in Spider surgical system,the surgeon inserts the SPIDER Surgical System through a single incision usually located near the patient's belly button. The system opens up umbrella-like within the abdomen, providing the surgeon with two flexible channels for right- and left-hand instruments with 360-degree range of motion, and two rigid channels for small cameras and other instruments. Once the procedure is completed, the SPIDER Surgical System closes up and is removed through the same incision.
The spider surgical system is presently used for appendectomy, gall bladder surgeries and mainly bariatric surgeries. With the periodic transition in the field of abdominal surgeries this Spider tech is said to be a giant leap..
Source: http://www.transenterix.com/spider-surgical-system.php
Monday, July 9, 2012
Electronic Resources in Medicine (ERMED) Consortium

1. American Academy of Pediatrics
2. BMJ Publishing
3. Cambridge University Press
4. Cengage Learning
5. IOS Press
6. Lippincott William Wilkins
7. Oxford University Press
8. Royal Society of Medicine Press
To join ERMED the following application is to be filled
http://nmlermed.in/Application.pdf
The applications should be sent to the address below
National Medical Library
Ansari Nagar,
Ring Road,
New Delhi-110029.
Ph: 011-26589085,26589128,26589489
e-mail: nationalmedicallibrary66@yahoo.in
http://nmlermed.in/howToJoin.htm
Most of the Govt. Medical colleges in India are lagging behind in Research and recent advances in the world of medicine because of various reasons. ERMED is an effort to help the medical colleges and students to update themselves with newer things.
For more details visit
Sunday, July 8, 2012
iPhone apps for Doctors
Since ages it’s a general perception that doctors are considered to be weak in technical things and poor in gadget using. Now the time has changed with , whole of the medical profession is being dependent on gadgets.The days of bedside teaching , learning and being proud of ones clinical skills will become like test cricket in years to come. Change is the principle of nature and in the struggle for existence the most adaptable will survive. Its becoming mandatory to use medical applications in our smart phones for quick response during emergencies.. Here are few medical applications for iPhone. The first one in the review series of the medical apps.
1. Heart pro
3D4Medical in collaboration with Stanford University School of Medicine present the Heart Pro III. As featured in the WWDC 2012 Keynote Speech. This app was developed in partnership with Dr. Lacy E Harville III, MD, FACS.
In this upgrade 3D4Medical have added in several new features and several new animations all for free.
Built in Animations:
-Anterior Beating Heart - Aortic Stenosis - Conduction Heart Beat - Heart Catheterization-Ischemic Stroke - Myocardial Heart Attack - Patent Ductus Arteriosus (PDA) - Ventricular Fibrillation - Ventricular Septal Defect - Blood Flow (slow speed) - Blood Flow in Beating Heart - Coronal Cut (Ant.) Beating Heart - Lateral Beating Heart - Posterior Beating Heart - Right Lateral Beating Heart - Sagittal Cut (Lat) Beating Heart - Transverse Cut (Ant) Beating Heart - Aortic Valve - Left Atrium - Left Ventricle - Mitral Valve - Pulmonary Valve - Right Atrium - RightVentricle Tricuspid Valve.
To buy the app visit
http://itunes.apple.com/us/app/heart-pro-iii/id393231526?mt=8